Clathrin Coat Dynamics in Vesicle Transport
Notes on Clathrin Coat Assembly and Disassembly
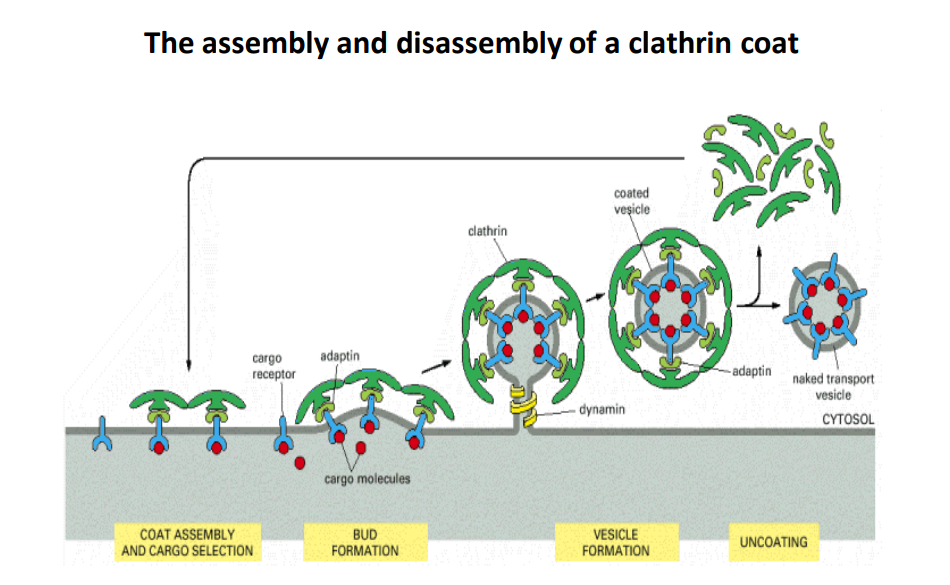
Overview of Clathrin Function
- Clathrin is a protein that plays a crucial role in the formation of vesicles, which are essential for transporting molecules within cells.
- The process involves two main phases: assembly and disassembly of the clathrin coat around the vesicles.
Steps in Clathrin Coat Process
1. Coat Assembly and Cargo Selection
- Cargo Receptors: These proteins recognize and bind to specific cargo molecules that need to be transported.
- Adaptin: This protein connects the cargo receptors to the clathrin coat, facilitating the selection of the right cargo.
- Thoughts: Efficient cargo selection is critical for cellular function because it ensures that the right materials are delivered to the proper cellular destinations.
2. Bud Formation
- Clathrin molecules assemble into a basket-like structure around the cargo-bound receptors and adaptins.
- The curvature of the clathrin coat helps to form a budding vesicle from the plasma membrane.
- Dynamin: This protein is involved in the final pinching off of the vesicle from the membrane.
- Thoughts: The process of bud formation is tightly regulated, and any malfunction can lead to issues such as impaired cell communication or nutrient uptake.
3. Vesicle Formation
- As the bud continues to grow and pinch off, it forms a complete vesicle coated in clathrin that encases the cargo.
- This vesicle is referred to as a coated vesicle.
4. Uncoating
- Once inside the cytosol, the vesicle undergoes uncoating, where the clathrin coat is removed to expose the naked transport vesicle.
- This step is crucial for the vesicle to fuse with its target membrane and deliver its contents.
- Thoughts: Uncoating must be efficient and timely to ensure proper delivery of cargo, as lingering coats could interfere with fusion processes.
Summary
- The assembly and disassembly of the clathrin coat are essential for intracellular transport. Each stage of the process involves specific proteins that work together to facilitate cargo recognition, vesicle formation, and eventual cargo delivery. Understanding these mechanisms can provide insights into cellular communication and transport systems.
Extended readings:
The Role of Dynamin in Clathrin-Coated Vesicle Formation
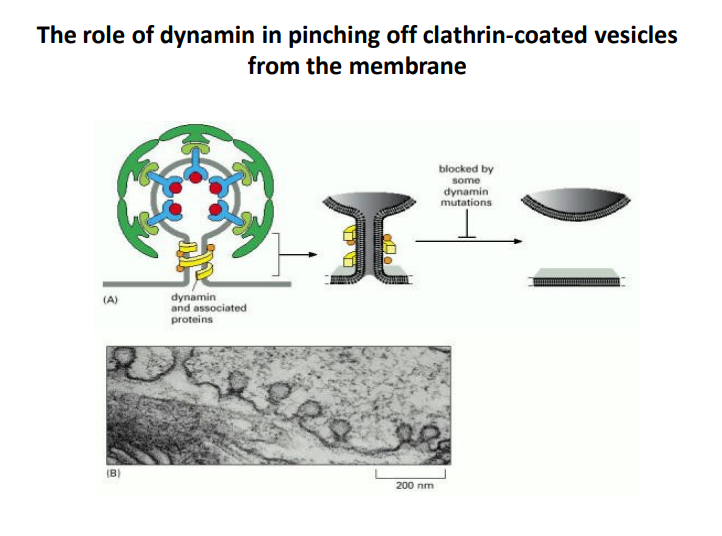
-
Dynamin Functionality
- Dynamin is a GTPase enzyme that plays a critical role in the final stages of vesicle formation. It is responsible for "pinching off" clathrin-coated vesicles from the membrane.
- This process involves the assembly of dynamin into a helical structure around the neck of the budding vesicle, which facilitates membrane fission.
-
Importance of Dynamin Mutations
- Some mutations in dynamin can block the pinching-off process, preventing vesicles from forming properly.
- This highlights the significance of dynamin in maintaining proper cellular functions, including endocytosis and signal transduction.
-
Clathrin-Coated Vesicles Formation
- The clathrin coat is essential for the recruitment of cargo and adaptation of the vesicle for transport.
- The image illustrates the sequential steps leading to the final release of the vesicle, emphasizing the role of dynamin in this intricate process.
-
Visual Representation
- Panel A depicts the structure of dynamin and associated proteins, which shows the helical arrangement essential for its function.
- Panel B provides a micrograph showing vesicles, which aids in visualizing the process discussed.
-
Significance in Cellular Processes
- Understanding how dynamin functions in vesicle formation aids in comprehending various cellular processes, including nutrient uptake, receptor recycling, and neurotransmitter release.
- Disruptions in this mechanism could lead to various diseases, including neurodegenerative disorders and metabolic syndromes.
By studying the interaction between dynamin and clathrin-coated structures, researchers can better understand and potentially target pathways involved in these critical cellular processes.
Extended readings:
A current model of COPII-coated vesicle formation
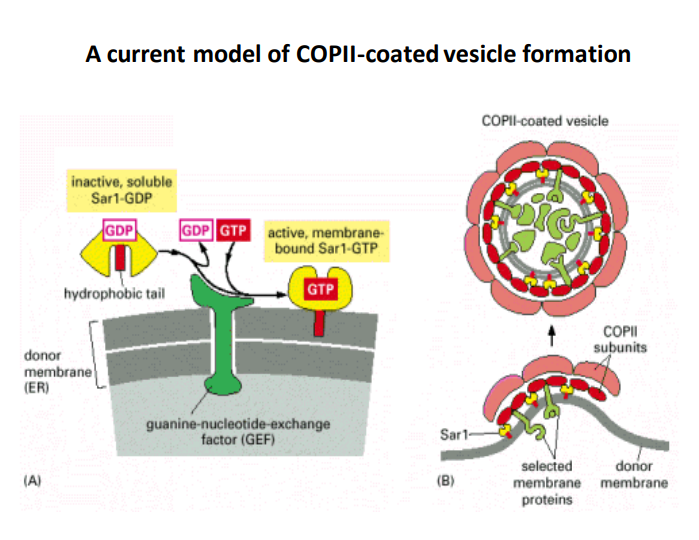
-
COPII-Coated Vesicles: These vesicles are essential for the transport of proteins from the endoplasmic reticulum (ER) to the Golgi apparatus. The COPII coat is made up of various proteins that regulate the budding process.
-
Sar1 Protein: Sar1 is a GTPase that plays a critical role in COPII vesicle formation.
- Inactive Form (Sar1-GDP) : In its inactive state, Sar1 is soluble and cannot interact with membranes or assist in vesicle formation.
- Activation: When GDP is exchanged for GTP, Sar1 becomes membrane-bound (Sar1-GTP), which is essential for the initiation of coat assembly on the ER membrane.
-
Guanine-Nucleotide-Exchange Factor (GEF) : This factor facilitates the exchange of GDP for GTP on Sar1. Its activity is crucial for activating Sar1 to promote vesicle formation.
- Importance: By influencing the activation state of Sar1, GEF plays a pivotal role in regulating the dynamics of COPII vesicle formation.
-
Hydrophobic Tail: The hydrophobic tail of Sar1 contributes to its localization to the ER membrane, allowing the protein to anchor and trigger vesicle assembly.
-
Vesicle Formation Process:
- Step 1: Sar1-GTP attaches to the donor membrane, encouraging the recruitment of other COPII coat proteins.
- Step 2: The COPII coat forms around the selected membrane proteins, which helps in concentrating and packaging cargo for transport.
- Step 3: Eventually, the vesicle buds off from the ER membrane, facilitated by the coat's structural dynamics.
-
Selected Membrane Proteins: The vesicle selectively includes certain proteins as cargo, which is critical for effective cellular function and transport to the Golgi apparatus.
-
COPII Subunits: These are the building blocks of the COPII coat, which play an important role in the stabilization and structure of the budding vesicle.
-
Overall Importance: Understanding the mechanism of COPII vesicle formation is essential, as it underpins the broader processes of protein sorting and trafficking within cells, influencing cellular communication and function significantly.
Extended readings:
The Role of SNAREs in Vesicular Transport
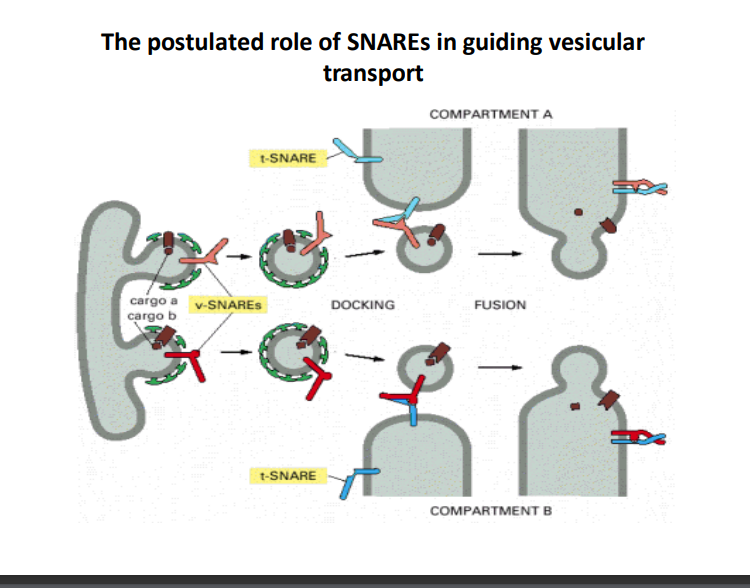
-
Overview of SNAREs
- SNARE (Soluble N-ethylmaleimide-sensitive factor Attachment protein REceptor) proteins are essential for the fusion of vesicles with their target membranes. There are two main types: v-SNAREs (vesicle SNAREs) and t-SNAREs (target SNAREs).
- Thoughts: Understanding the mechanism of SNARE proteins is crucial for comprehending intracellular transport processes, which are vital for cellular functionality.
-
Vesicular Transport Process
- Docking: v-SNAREs on the vesicle interact with t-SNAREs on the target compartment. This specific interaction helps ensure that vesicles fuse only with the correct membranes, allowing accurate cargo delivery.
- Additional Info: Misfiring in this process can lead to cellular dysfunction, as proteins may end up in incorrect locations.
-
Fusion Mechanism
- Once the vesicle is docked, SNARE proteins catalyze the fusion of the vesicle with the target compartment. This process involves the merging of lipid bilayers, allowing the cargo to transfer from the vesicle to the target compartment.
- Thoughts: This fusion process is highly regulated and is essential for many cellular functions, including neurotransmitter release and hormone secretion.
-
Role of Compartment A and Compartment B
- The diagram illustrates transport from Compartment A to Compartment B, highlighting that vesicular transport is directional and specific.
- Additional Info: Understanding compartment specificity aids in deciphering cellular organization and function across different organelles.
-
Importance in Cellular Communication
- The SNARE-mediated transport is fundamental for various signaling pathways within cells, impacting processes like metabolism, growth, and response to stimuli.
- Thoughts: Disruptions in SNARE function can lead to diseases, making them potential therapeutic targets.
| Feature | Description |
|---|---|
| v-SNARE | Located on the vesicle; specific to cargo |
| t-SNARE | Located on the target membrane; binds with v-SNARE |
| Docking | Initial interaction of SNAREs initiating vesicle fusion |
| Fusion | Merging of vesicle and target membrane allowing cargo release |
| Compartments | Specific areas within the cell facilitating organized transport |
This structured approach helps clarify the significance and mechanisms behind SNAREs in cellular transport, reinforcing their role in maintaining cellular integrity and communication.
Extended readings:
SNARE Pair Dissociation by NSF
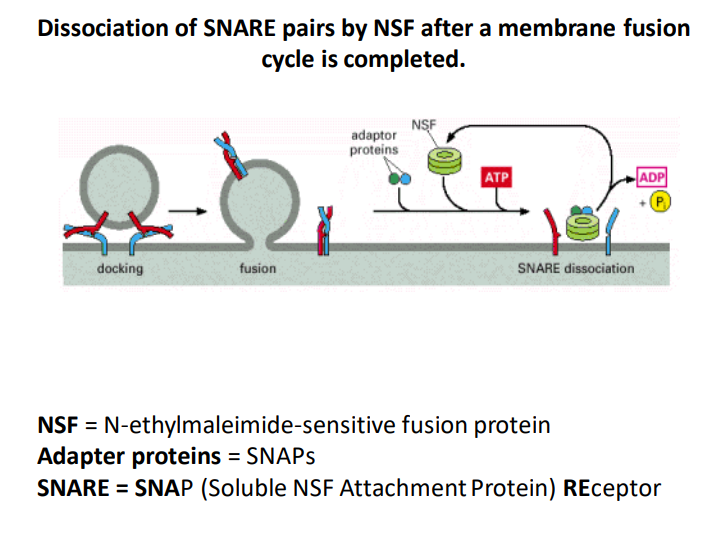
-
Membrane Fusion Cycle
The image illustrates the process of dissociating SNARE pairs by NSF (N-ethylmaleimide-sensitive fusion protein) after the completion of a membrane fusion cycle. This cycle is critical for intracellular communication and transport. -
Key Components
-
NSF: This protein is essential for the disassembly of SNARE (Soluble NSF Attachment Protein) complexes, allowing recycling of SNAREs for subsequent rounds of fusion.
Thoughts: Understanding how NSF operates can help elucidate mechanisms of synaptic transmission, where rapid recycling of SNAREs is crucial. -
Adapter Proteins (SNAPs) : These proteins assist NSF in facilitating SNARE complex disassembly. They help stabilize the interaction between NSF and SNARE complexes.
Ideas: Exploring different types of adapter proteins could provide insights into their role in various cellular processes beyond membrane fusion.
-
-
Process Summary
- Docking: The initial phase where vesicles brought by transport proteins attach to the target membrane.
- Fusion: SNARE proteins mediate the merging of the vesicle membrane with the target membrane to release contents into the cell or extracellular space.
- SNARE Dissociation: Following fusion, NSF utilizes ATP to dissociate the SNARE complexes into their components (SNAREs and SNAPs), enabling them to be reused in new rounds of vesicle fusion.
Additional Info: The conversion of ATP to ADP during this process releases energy that drives the dissociation.
-
ATP and ADP Cycle: The use of ATP and its conversion to ADP is a vital part of this mechanism as it provides the energy necessary for the dissociation of SNARE complexes, emphasizing the energy-dependent nature of cellular processes.
Thoughts: Exploring ATPase functions in cellular mechanics could enrich our understanding of metabolic influences on membrane dynamics.
Extended readings:
The Recruitment of Cargo Molecules into ER Transport Vesicles
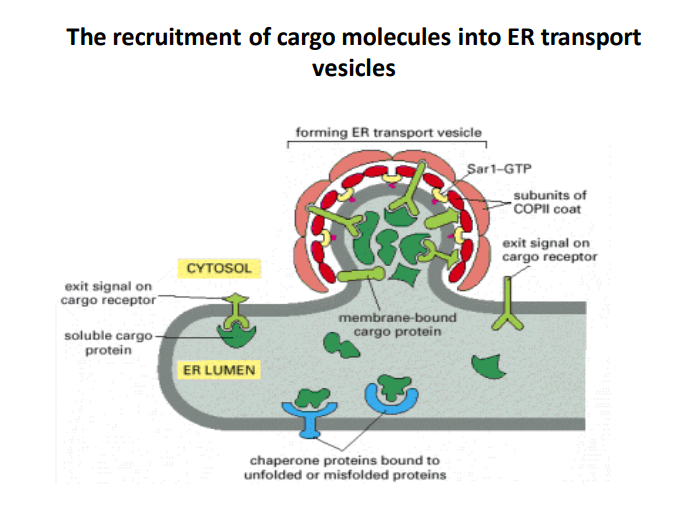
-
Formation of ER transport vesicles
The image illustrates the process of vesicle formation at the endoplasmic reticulum (ER), highlighting the role of Sar1-GTP in initiations. Understanding this is crucial due to the ER's importance in synthesizing and transporting proteins. -
Role of COPII coat subunits
The subunits of the COPII coat are vital for the recruitment of cargo molecules into vesicles. They provide structural support and facilitate the budding process. COPII-coated vesicles specifically transport proteins from the ER to the Golgi apparatus, a key step in the secretory pathway. -
Cargo Receptors with Exit Signals
The presence of exit signals on cargo receptors is critical for selecting the appropriate cargo molecules for vesicle transport. These signals ensure that only correctly folded and properly assembled proteins are packaged, preventing misfolded proteins from exiting the ER. -
Chaperone Proteins Function
Chaperone proteins bind to unfolded or misfolded proteins, facilitating proper folding and quality control in the ER lumen. This process prevents defective proteins from entering the transport pathway, thereby maintaining cellular health. -
Cytoplasm and ER Lumen Interaction
The dynamic interaction between the cytosol and the ER lumen is essential for cargo selection and vesicle formation. It highlights how cellular compartments work together to ensure efficient protein transport.
Table of Components in the Vesicle Recruitment Process
| Component | Function |
|---|---|
| Sar1-GTP | Initiates the formation of ER transport vesicles |
| COPII coat subunits | Provide structure and facilitate budding of transport vesicles |
| Cargo receptors | Bind to cargo molecules with exit signals for selective transport |
| Chaperone proteins | Aid in the proper folding of proteins and prevent misfolded proteins |
| Soluble cargo proteins | Proteins that need to be transported from the ER to Golgi |
| Membrane-bound cargo proteins | Proteins associated with the membrane that require transport |
| Exit signals | Specific signals that indicate readiness for transport |
Extended readings:
A Model for the Retrieval of ER Resident Proteins
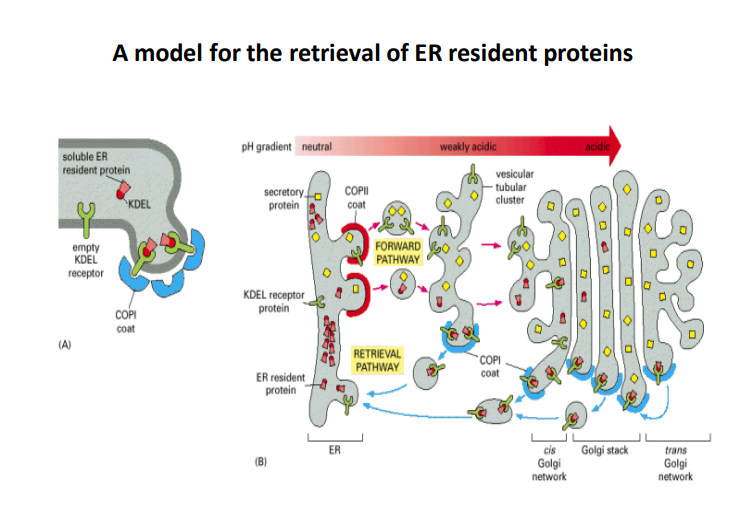
-
ER Resident Proteins
- These proteins are essential for maintaining proper functions within the endoplasmic reticulum (ER). They can be retrieved from the Golgi apparatus back to the ER as needed to preserve homeostasis and functionality.
-
KDEL Receptor
- The KDEL receptor specifically recognizes proteins containing the KDEL sequence at their C-terminus. This sequence is crucial for the retrieval process.
- It binds to the soluble ER resident proteins that inadvertently leave the ER and facilitates their return.
-
COPI and COPII Coats
- COPII Coat: Involved in the forward transport of proteins from the ER to the Golgi apparatus. It plays a role in budding off vesicles from the ER.
- This coat is essential for the selection and packaging of proteins aimed for secretion.
- COPI Coat: This coat is responsible for retrograde transport, retrieving resident ER proteins from the Golgi back to the ER.
- It ensures that proteins necessary for ER function are recycled effectively.
- COPII Coat: Involved in the forward transport of proteins from the ER to the Golgi apparatus. It plays a role in budding off vesicles from the ER.
-
Forward Pathway
- This pathway describes the movement of secretory proteins and their initial processing in the Golgi. Key steps include:
- Packaging into COPII-coated vesicles for transport to the Golgi apparatus.
- Proteins are modified and sorted for their final destination.
- This pathway describes the movement of secretory proteins and their initial processing in the Golgi. Key steps include:
-
Retrieval Pathway
- This pathway is critical for bringing back ER resident proteins that escape to the Golgi. It involves:
- The binding of KDEL receptor proteins to their ligands.
- The formation of COPI-coated vesicles that transport these proteins back to the ER.
- This pathway is critical for bringing back ER resident proteins that escape to the Golgi. It involves:
-
pH Gradient
- The pH gradient across the ER and Golgi apparatus is influential in protein sorting and binding affinity.
- For instance, the acidic environment of the Golgi enhances the binding of KDEL receptor proteins to their ligand.
-
Cis and Trans Golgi Networks
- The Golgi apparatus is organized into distinct functional compartments (cis and trans).
- Cis Golgi Network: Receives proteins from the ER and is involved in early processing.
- Trans Golgi Network: Involved in the sorting and dispatching of proteins to their final destinations.
| Component | Function |
|---|---|
| Soluble ER resident protein | Maintains ER functions; must be retrieved |
| KDEL receptor | Binds to KDEL proteins for retrieval |
| COPII coat | Mediates forward transport to Golgi |
| COPI coat | Mediates retrieval from Golgi to ER |
| pH gradient | Influences protein binding in tissues |
| Cis Golgi network | Processes incoming proteins |
| Trans Golgi network | Sorts proteins for final destinations |
This model highlights the dynamic processes involved in maintaining ER protein homeostasis, emphasizing the significance of various protein interactions and transport mechanisms in cellular physiology.
Extended readings: