science
Light

Basic Principles
- Light Travel: Light travels in straight lines in the same medium.
- Absorption: Some light may be absorbed into an object, transforming energy into heat.
- Reflection: The law of reflection states that:
- Angle of Incidence (i) = Angle of Reflection (r) .
- The normal line is an imaginary line drawn perpendicular to an object's surface.
- Angles are always measured between the ray and the normal.
- Angle of Incidence (i) = Angle of Reflection (r) .
Reflection Types
- Specular Reflection: Reflection off a smooth surface where light rays are reflected at consistent angles, producing a clear image.
- Diffuse Reflection: Reflection from a rough surface, scattering light rays in various directions, resulting in no clear image.
Refraction
- Definition: Refraction is the bending of light as it travels from one medium to another.
- Densities:
- If light transmits into a denser medium, it slows down and bends towards the normal.
- If light transmits into a less dense medium, it speeds up and bends away from the normal.
- Densities:
Diagram
- ![Refraction Diagram]
Incoming Rayx\\\\Normal\\\Reflected Ray
Additional Insights
- Importance of Normal Line: The normal line is crucial in calculating angles in reflection and refraction.
- Applications: Understanding light behavior is vital in optics, photography, and designing lenses.
Note: Always consider how light interaction with different mediums can affect visibility and clarity of images in real-world applications.
Extended readings:
Total Internal Reflection and Mirrors
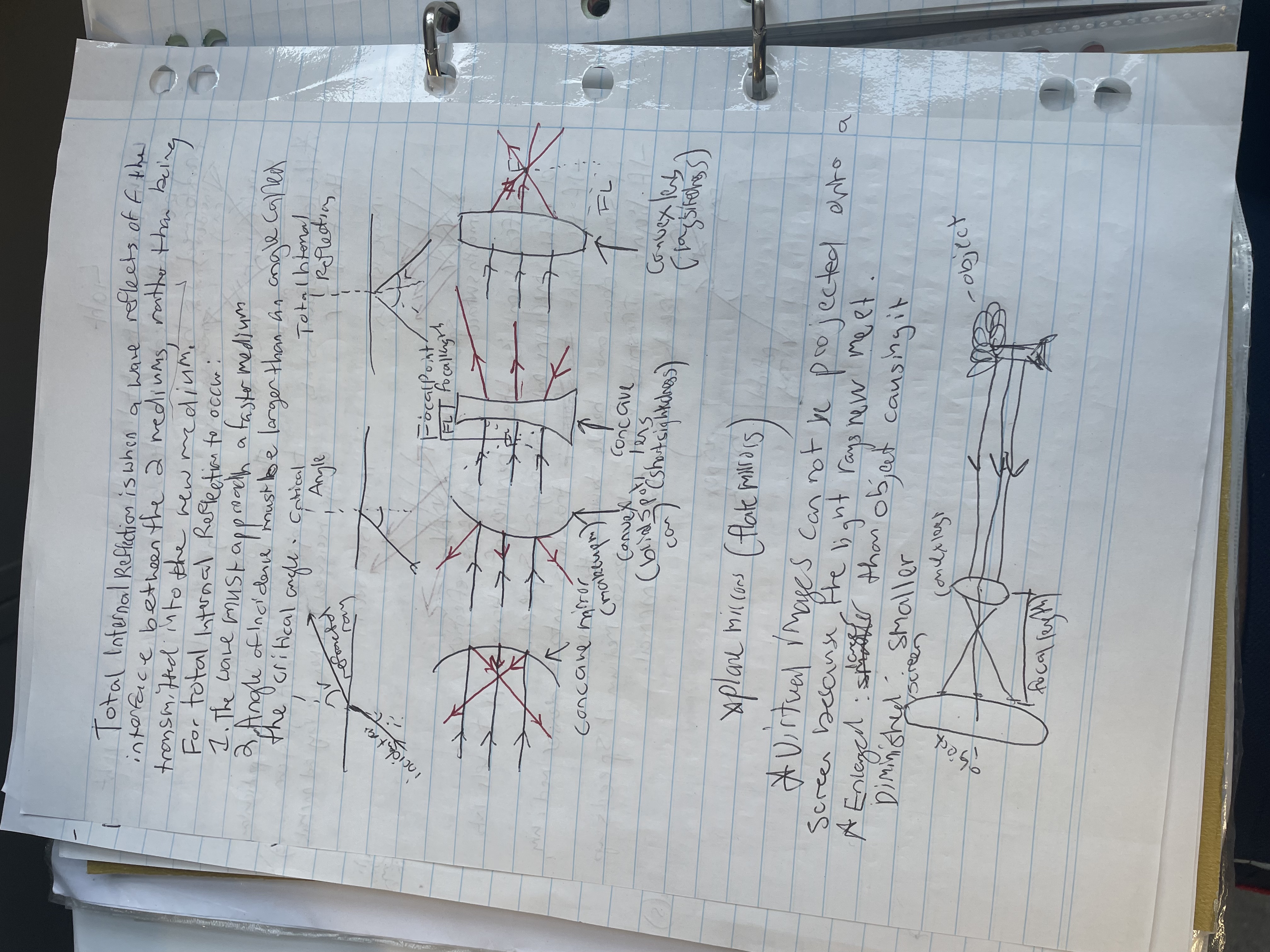
Total Internal Reflection
- Definition: Total internal reflection occurs when a wave reflects off the interface between two media, rather than being transmitted into the second medium.
- Conditions for Occurrence:
- The wave must approach a faster medium.
- The angle of incidence must be greater than the critical angle.
Critical Angle
- Definition: The critical angle is the minimum angle of incidence at which total internal reflection occurs.
- Insight: This concept is essential in optics, especially in fiber optics, where light is guided through total internal reflection.
Focal Points and Mirrors
- Concave Mirrors:
- Function: Converge light rays to a focal point.
- Diagram Features:
- Incident Rays: Lines representing light approaching the mirror.
- Focal Length (FL) : The distance from the mirror's surface to the focal point.
Aplanatic Mirrors (Flat Mirrors)
- Definition: These mirrors do not alter the shape of the incoming wavefront.
- Key Characteristics:
- Virtual Images: Cannot be projected onto a screen since the light rays do not actually converge.
- Enlargement: Virtual images are often larger than the object causing them and can be diminished or smaller, based on the object's distance from the mirror.
Additional Terms
- Focal Length (FL) : The distance from the mirror to the focal point where parallel rays converge.
- Virtual Image: An image formed by rays that appear to diverge from a point, but do not actually converge there (e.g., images seen in mirrors).
Summary
These notes cover fundamental aspects of optics, including total internal reflection and the characteristics of concave and flat mirrors. Understanding these principles is crucial for applications in lens design, optics in photography, and various technologies relying on light manipulation.
Extended readings:
Chemistry Notes

Basic Concepts
-
Atom
- Definition: The smallest unit of matter that retains the properties of that matter.
- Structure: Composed of a nucleus (containing protons and neutrons) and electrons orbiting around it.
-
Element
- Definition: A substance made from only one type of atom.
- Examples: Oxygen (O), Carbon (C), and Gold (Au).
-
Atomic Structure
- Atoms consist of:
- Protons (positively charged)
- Neutrons (neutral)
- Electrons (negatively charged)
- Maximum number of electrons in each shell can be calculated using the formula , where n is the shell level (e.g., 1, 2, 3).
- Atoms consist of:
-
Mass and Atomic Number
- Atomic Mass: The weighted average mass of an element's isotopes.
- Atomic Number: The number of protons in the nucleus of an atom, which determines the element's identity.
Periodic Table Groups
-
Group 1: Alkali Metals
- Highly reactive, particularly with water.
- Examples: Lithium (Li), Sodium (Na).
-
Group 2: Alkaline Earth Metals
- Reactive metals but less so than alkali metals.
- Examples: Magnesium (Mg), Calcium (Ca).
-
Group 17: Halogens
- Highly reactive nonmetals.
- Commonly used in disinfection (e.g., Chlorine, Fluorine).
-
Block between Groups 2 and 3: Transition Metals
- Known for variable oxidation states and colored compounds.
- Include elements like Iron (Fe) and Copper (Cu).
Chemical Bonding
-
Ionic Bonds
- Involve the transfer of electrons from one atom to another.
- Form between a metal and a non-metal.
- Ions: Formed when atoms gain or lose electrons.
- Cations: Positively charged ions (lost electrons).
- Anions: Negatively charged ions (gained electrons).
-
Covalent Bonds
- Involve sharing electrons between atoms.
- Typically form between non-metals.
- Follow the Octet Rule: Atoms tend to form bonds until they are surrounded by eight valence electrons, achieving stable electronic configurations.
Summary of Key Points
- Electrons can be transferred from a metal atom to a non-metal atom during ionic bonding.
- The bond formed when an atom gains or loses an electron is known as an ionic bond.
- Ionic compounds are typically structured in lattices, which enhances stability.
Additional Insights
Understanding the periodic table and the types of chemical bonds is fundamental in predicting the behavior of elements and their compounds in chemical reactions. Keeping these basic principles in mind can aid in mastering more complex topics in chemistry.
Extended readings:
Covalent Compounds and Acids & Bases

Covalent Compounds
-
Definition: Covalent compounds form when electrons are shared between non-metal atoms. Each covalent bond consists of two shared electrons, one from each atom. The group of negatively charged atoms is called a molecule.
- Insight: Understanding electron sharing is critical in chemistry, as it leads to the formation of stable compounds through covalent bonding.
- Examples: Common diatomic molecules include H₂ (hydrogen) and F₂ (fluorine), where two identical atoms are covalently bonded.
-
Diatomic Molecules:
- Definition: Diatomic molecules are specific to two identical atoms covalently bonded together.
- Examples:
- H₂: Hydrogen
- F₂: Fluorine
Acids & Bases
-
Neutralization Reaction: When an acid meets a base, a neutralization reaction occurs. This reaction typically results in the formation of water and a salt.
- Insight: Neutralization is a fundamental chemical reaction that illustrates the concept of acid-base chemistry, where the properties of two reactants change upon interaction.
-
Characteristics of Acids and Bases:
- Acids:
- Generally have a sour taste.
- Are considered corrosive, meaning they can damage or destroy tissues.
- Bases:
- Often taste bitter and feel slippery.
- Are considered caustic, which means they can burn or corrode organic tissues.
- In Water:
- Acids tend to produce hydrogen ions (H⁺) in aqueous solution, which is responsible for their reactivity.
- Bases produce hydroxide ions (OH⁻), contributing to their basicity.
- Acids:
Summary Table of Acids and Bases
| Property | Acids | Bases |
|---|---|---|
| Taste | Sour | Bitter |
| Feel | N/A | Slippery |
| Chemical Reactions | Produce H⁺ ions | Produce OH⁻ ions |
| Corrosive Nature | Yes | Yes |
- Key Reaction: The reaction between an acid and a base results in a neturalization process leading to the production of water and a salt.
This structured overview provides a comprehensive understanding of covalent compounds and the fundamental properties of acids and bases.
Extended readings: