Transport Mechanisms of Drugs Across Membranes
Transport of Drugs Across Biological Membranes

Overview
Mermaid flowchart of transport mechanisms:
graph TDA[Transport of Drugs] --> B[Passive Diffusion]A --> C[Filtration]A --> D[Specialized Transport]D --> E[Carrier Mediated]D --> F[Vascular Transport]E --> G[Facilitated Diffusion]E --> H[Active Transport]H --> I[Primary Active Transport]H --> J[Secondary Active Transport]F --> K[Endocytosis]F --> L[Exocytosis]
I. Passive Diffusion
-
Movement:
- Drugs move from high to low concentration.
- Insight: This process is driven by the concentration gradient, crucial for maintaining homeostasis.
-
Characteristics:
- No carrier involvement, non-saturable, low specificity.
- Explanation: Unlike active transport, it does not require energy or specific transport proteins.
-
Rate of Transport:
- Proportional to the lipid: water partition coefficient.
- Insight: Lipid solubility increases diffusion rate as cell membranes are lipid bilayers.
-
Solubility:
- Lipid-soluble drugs achieve higher membrane concentration and diffuse faster.
- Note: Highly lipid-soluble drugs pass through membranes more easily, important for drug design.
-
Concentration Gradient Effect:
- Greater concentration difference leads to faster diffusion.
- Insight: Steeper gradients enhance the passive diffusion rate, critical in pharmacokinetics.
II. Filtration
-
Process:
- Passage of drugs through aqueous pores.
- Explanation: Utilizes openings in the membrane to facilitate passage.
-
Drug Characteristics:
- Water-soluble drugs with molecular size < 100 molecular weight and smaller than pore diameter (7Å).
- Insight: Molecular size and solubility are crucial in determining the filtration mechanism's effectiveness.
This concise summary provides a clear understanding of the mechanisms for drug transport across biological membranes, emphasizing the key elements and implications for pharmaceutical applications.
Extended readings:
Specialized Transport Mechanisms

Carrier Mediated and Vesicular Transport
1. Carrier Transport
-
Definition: Movement of substances that cannot diffuse directly, aided by carrier proteins.
- Insight: This process is vital for transporting specific molecules like glucose and ions across cell membranes.
-
Characteristics:
- Specificity: Carrier transport is specific to the substrate.
- Explanation: Each type of carrier protein is tailored to handle a specific molecule or group of molecules.
- Saturable: Can reach a transport maximum when all carriers are occupied.
- Competitive Inhibition: Presence of similar molecules can inhibit transport.
- Example: Drugs or molecules with similar structure can compete for the same carrier.
- Slower Rate: Typically slower than diffusion through pores or channels.
- Specificity: Carrier transport is specific to the substrate.
-
Types Based on Energy Requirement:
- Facilitated Diffusion:
- Energy Independent: Does not require energy input.
- Mechanism: Carrier proteins undergo conformational changes to transport molecules.
- Direction: Moves substances from high to low concentration.
- Examples: Uptake of glucose and vitamin B₁₂.
- Active Transport:
- Energy Dependent: Requires energy (usually ATP) to function.
- Mechanism: Transports molecules against their concentration gradient.
- Examples: Transport of levodopa, iron, sugars, and amino acids.
- Source of Driving Force: Energy source determines the nature of active transport.
- Facilitated Diffusion:
Diagrams
-
Facilitated Diffusion Diagram:
graph TBA(Drug) -->|High Concentration| B[Carrier Protein]B -->|Conformation Change| C[Low Concentration] -
Active Transport Diagram:
graph TBD(Drug) -->|Low Concentration| E[Carrier Protein]E -->|Energy from ATP| F[High Concentration]
These mechanisms are crucial in pharmacology for understanding how drugs and metabolites are transported within the body, impacting their effectiveness and distribution.
Extended readings:
Transport Mechanisms Across Cell Membranes
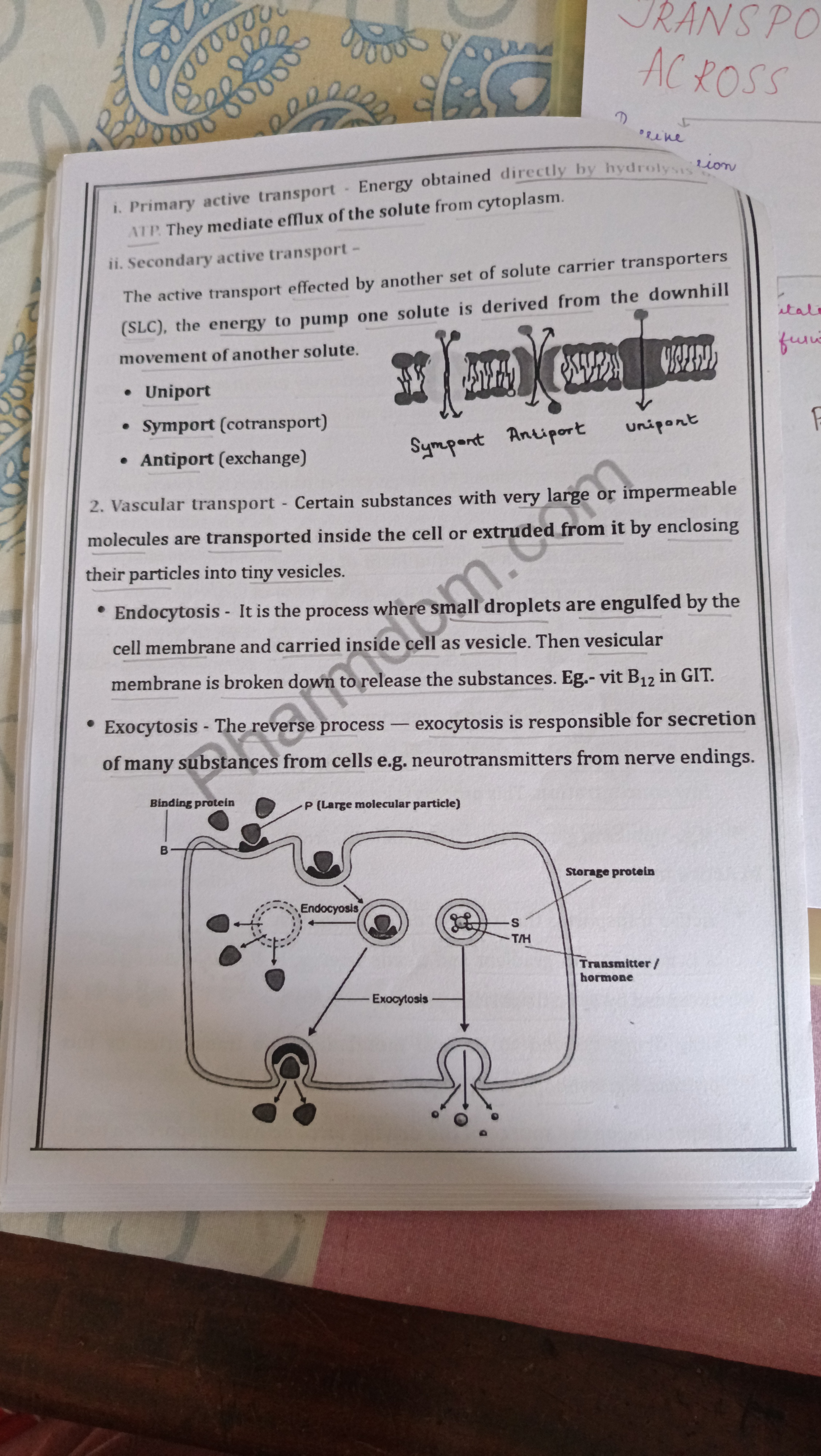
1. Primary Active Transport
- Definition: Energy is obtained directly by hydrolysis of ATP.
- Function: Mediates the efflux of solutes from the cytoplasm.
- Insight: This process moves substances against their concentration gradient, essential for maintaining cellular homeostasis. ATPases like the sodium-potassium pump are key examples.
2. Secondary Active Transport
- Mechanism: Utilizes energy from the movement of another solute.
- Types:
- Unimport: Transports a single solute across the membrane.
- Symport (Cotransport) : Moves two solutes in the same direction.
- Antiport (Exchange) : Moves two solutes in opposite directions.
- Insight: In secondary active transport, the energy comes from the electrochemical gradient created by primary active transport, not directly from ATP. Examples include the glucose-sodium symport and the sodium-calcium exchanger.
3. Vascular Transport
- Definition: Transports substances by enclosing them in vesicles.
- Types:
- Endocytosis: Engulfs large molecules via the cell membrane, forming vesicles.
- Example: Uptake of vitamin B₁₂ in the gastrointestinal tract.
- Insight: Helps cells internalize molecules like nutrients and pathogens for cellular processes.
- Exocytosis: Secretes substances from the cell, such as neurotransmitters.
- Insight: Critical for processes like neurotransmitter release in neurons.
- Endocytosis: Engulfs large molecules via the cell membrane, forming vesicles.
Flowchart: Endocytosis and Exocytosis
flowchart TDA(Outside Cell) -->|Endocytosis| B(Vesicle Formation)B --> C(Inside Cell)D(Inside Cell) -->|Exocytosis| E(Vesicle Fusion)E --> F(Outside Cell)
- Additional Note: Vesicular transport mechanisms are crucial for maintaining cellular communication and material exchange with the environment. They allow cells to interact with their surroundings dynamically.
Extended readings:
Introduction to Medicinal Chemistry

Overview
Medicinal Chemistry
- Definition: Medicinal chemistry combines aspects of chemistry, pharmacology, and biology for the design and development of pharmaceutical agents.
- Importance: Central to drug discovery and development, focusing on the chemical aspects of drug activity and metabolism.
History & Development
Early Beginnings
- Ancient Practices: Use of natural substances (herbs, minerals) in healing practices dates back thousands of years.
- Alchemy: Medieval roots with the transformation of base materials into valuable substances, laying grounds for modern chemistry.
Modern Era
- 19th Century: Emergence of organic chemistry; isolation of active ingredients from plants.
- 20th Century: Advancements in understanding molecular structures led to the synthesis of new drugs.
- Technological Innovations: High-throughput screening, computational modeling, and biotechnology revolutionize drug design.
Key Milestones
- Isolation of Morphine (1804) : Provided insight into the active components of medicinal plants.
- Synthesis of Aspirin (1897) : Highlighted the potential of chemical synthesis in creating effective drugs.
- Discovery of Penicillin (1928) : Marked the beginning of antibiotics and modern therapeutic practices.
Educational Pathways
Muhammad Nizam V P
- Academic Pursuit: Pursuing a master's degree at NIPER Hyderabad, focusing on the specialized field of medicinal chemistry.
Conclusion
Medicinal chemistry is a dynamic and interdisciplinary field fundamental to the innovation of new therapeutics. The integration of historical practices with modern technologies continues to drive advancements in health care.
Note: This summary provides insights into the foundational concepts and evolution of medicinal chemistry to aid in understanding its significance and applications.
Extended readings: