Chapter 6: Understanding Chemical Bonds and Molecules
Chapter 6: Chemical Bonds

Overview
- Electrons and Atom Union: Electrons occupy the outer shell of an atom. Typically, atoms are more stable when their outer shells are full. Therefore, atoms often bond together to either share or exchange electrons to achieve a stable electronic structure.
- Ionic and Covalent Bonds:
- Ionic Bonds: Formed when electrons are transferred from one atom to another, resulting in positive and negative ions that attract each other.
- Covalent Bonds: Occur when atoms share electrons to fill their outer shells.
Molecules and Chemical Formulas
- Molecules Formation: When atoms bond, they form molecules, which can vary in shape and size.
- Molecular Representation:
- Represent molecules by placing symbols together (e.g., H2O for water).
Examples of Molecules
-
Hydrogen Molecule (H₂O) :
- Structure: Composed of two hydrogen atoms and one oxygen atom.
- Bond Type: Covalent.
- Insights: Common example of a covalent bond, demonstrating how atoms share electrons.
-
Oxygen Molecule (O₂) :
- Structure: Composed of two oxygen atoms.
- Bond Type: Double bond.
- Insights: Essential for respiration, this molecule illustrates how molecules can bond using different numbers of bonds.
-
Nitrogen Molecule (N₂) :
- Structure: Composed of two nitrogen atoms.
- Bond Type: Triple bond.
- Insights: This strong bond is key to the stability of nitrogen in the atmosphere.
Electron Configuration and Stability
- Octet Rule: Atoms tend to be more stable when they have eight electrons in their outer shell. Exceptions include hydrogen and helium, which are stable with two.
Did You Know?
- Neon Lights:
- Concept: Neon lights emit light when a high voltage is applied. This excites the electrons, which release energy in the form of light when returning to their ground state.
- Application: Used in signage and decorative lighting, showcasing practical applications of electronic excitations and emissions.
Additional Insights
- Bond Energy: Different bonds have varying energy levels, impacting the stability and reactivity of molecules.
- Chemical Reactions: Bonds are broken and formed during chemical reactions, highlighting the dynamic nature of molecular interactions.
Related Concepts
- Valency: The combining capacity of an element, crucial for predicting molecule formation.
- Noble Gases: Known for full outer shells, making them largely unreactive, which provides a reference point for understanding bond formation in other elements.
Extended readings:
Chapter 6: Chemical Bonds

What are Ions?
-
Definition: Ions are atoms or molecules that have gained or lost one or more electrons, resulting in a net charge.
- Cations: Positively charged ions (e.g., metals like Potassium losing electrons).
- Anions: Negatively charged ions (e.g., Fluorine gaining electrons).
-
Importance of Ions: Ions play a crucial role in chemical reactions by participating in the bond formation between different elements.
Insights:
- Ions are fundamental in creating ionic bonds, which are formed between metals and non-metals.
- Understanding ion formation helps in predicting the reactivity and properties of elements.
Combining Atoms and Valency
-
Chemical Bonds: Atoms combine or bond during reactions to fill their outer shells. The goal is stability, usually achieved with a full outer shell.
-
Valency: Indicates how many electrons an atom will gain, lose, or share to achieve a stable configuration.
- Elements with similar valency often exhibit similar chemical behaviors.
Insights:
- The concept of valency is crucial for understanding how compounds like H₂O (water) and CO₂ (carbon dioxide) form.
- Valency guides the prediction of molecular structures and helps in balancing chemical equations.
Additional Information
-
Valency often correlates with the group number in the periodic table. For example, Group 1 elements typically have a valency of 1.
-
Fluorine Example: As a halogen, fluorine is highly reactive due to its strong electron affinity, often forming negative ions.
Valency Table
| Group Number in Periodic Table | Number of Electrons in Outer Shell | Valency |
|---|---|---|
| 1 | 1 | 1 |
| 2 | 2 | 2 |
| 3 | 3 | 3 |
| 4 | 4 | 4 |
| 5 | 5 | 3 |
| 6 | 6 | 2 |
| 7 | 7 | 1 |
| 0 | 8 (full shell) | 0 |
Insights:
- The table helps visualize how the periodic table organizes elements by their valency, assisting in predictions of element behavior in reactions.
- The concept of electron configuration is simplified by grouping elements into these categories.
Understanding ions and valency builds a solid foundation for exploring more complex chemistry topics, such as molecular geometry and chemical kinetics.
Extended readings:
Chapter 1: Chemical Bonds
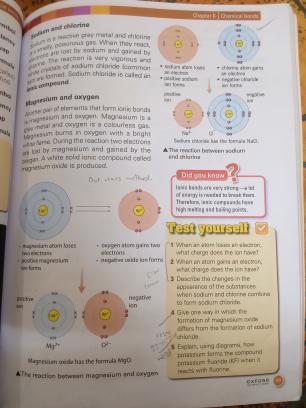
Bonding between Sodium and Chlorine
-
Reaction: Sodium (Na) and chlorine (Cl) form an ionic bond.
- Ionic Bond: An electrostatic attraction between oppositely charged ions, formed when atoms transfer electrons.
- Sodium loses one electron, becoming a positive ion (Na⁺).
- Chlorine gains one electron, becoming a negative ion (Cl⁻).
-
Compound Formation:
- The result is sodium chloride (NaCl), a stable compound.
- Significance: Sodium chloride, commonly known as table salt, is essential for human life and various industrial processes.
Bonding between Magnesium and Oxygen
-
Reaction: Magnesium (Mg) and oxygen (O) form an ionic bond.
- Magnesium loses two electrons, becoming a Mg²⁺ ion.
- Oxygen gains two electrons, becoming an O²⁻ ion.
-
Compound Formation:
- The compound formed is magnesium oxide (MgO).
- Properties: Used as a refractory material and in various industrial applications due to its stability.
Did You Know?
- Salt Uses: Beyond flavoring, salt is crucial for preserving food and plays a vital role in biological functions.
Test Yourself
Questions
-
Atoms and Ions:
-
What happens to an atom when it becomes a positive ion?
Insight: It loses electrons. Understanding electron transfer helps predict the behavior of ions in reactions.
-
-
Electron Transfer:
-
When an atom gains an electron, it becomes a negative ion.
Insight: Gaining electrons results in negative ion formation, which is key in ionic bonding.
-
-
Ion Charges:
- If an ion has a charge of 2+ (e.g., Mg²⁺), it has lost two electrons.
-
Compound Formation:
- Sodium and chlorine atoms combine to produce sodium chloride (NaCl).
Quiz Insight
- Practicing these questions enhances understanding of ionic bonding and electron exchange, foundational concepts in chemistry.
Extended readings:
Formation of Water (Hydrogen Oxide)
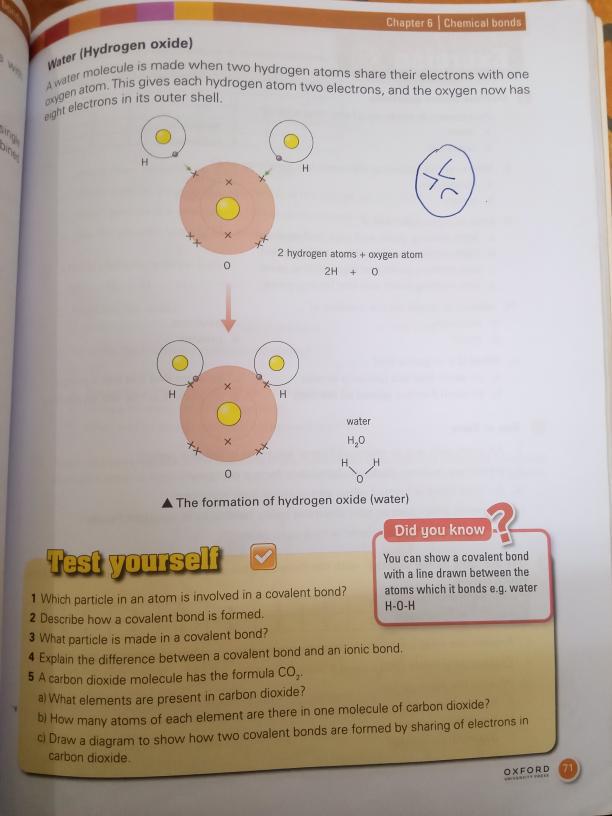
A water molecule is formed when two hydrogen atoms share their electrons with one oxygen atom.
-
Covalent Bonding in Water:
- Electron Sharing: Hydrogen atoms share electrons with oxygen, resulting in each hydrogen having two electrons and the oxygen having eight electrons in its outer shell.
- Diagram Representation: Water (H₂O) can be represented structurally to show the covalent bonds with lines (H-O-H).
-
Equation:
2H + O → H₂O- This represents the combination of two hydrogen atoms and one oxygen atom to form water.
Test Yourself Questions
-
Which particle in an atom is involved in a covalent bond?
- Electrons are involved in forming covalent bonds as they are shared between atoms.
-
Describe how a covalent bond is formed.
- Covalent Bond Formation: Involves sharing of electrons between atoms to achieve stability by filling their outer electron shells.
-
What particle is made in a covalent bond?
- Molecule: When atoms, like in water, share electrons, they form a molecule (e.g., H₂O).
-
Difference between a covalent bond and an ionic bond:
- Covalent Bond: Involves sharing of electrons between atoms.
- Ionic Bond: Involves the transfer of electrons from one atom to another, resulting in charged ions.
-
Carbon Dioxide Molecule (CO₂):
- Elements present: Carbon (C) and Oxygen (O).
- Number of each element: One carbon atom and two oxygen atoms per molecule of carbon dioxide.
Additional Insights:
- Covalent Bond Representation: Arrows or lines represent shared electron pairs.
- Octet Rule Insights: Atoms bond to fill their outer electron shells, achieving a stable configuration.
- Electron Sharing Vs. Transfer: Recognize the difference to understand molecular structures and compounds.
Did You Know?
- Drawing Covalent Bonds: Covalent bonds are depicted with lines connecting the atoms that share electrons (e.g., H-O-H for water).
By understanding these basic concepts of covalent bonds and molecular formation, you can dive deeper into the subject of chemical bonding, observing how molecules interact and form through electron sharing.
Extended readings:
Chemical Bonds Exercise

Multiple Choice Questions
-
An element is made up of only one type of...
- a. atom
- b. chemical
- c. molecule
- d. compound
Insight: Atoms are the basic units of elements. Molecules and compounds consist of two or more atoms bonded together. Elements in their pure form are composed solely of one type of atom.
-
Which of the following elements has a valency of 4?
- a. aluminium
- b. boron
- c. carbon
- d. nitrogen
Insight: Carbon typically has a valency of 4, meaning it can form four covalent bonds with other atoms. It's fundamental in organic chemistry.
-
Ionic compounds have a...
- a. high melting point and high boiling point
- b. high melting point and low boiling point
- c. low melting point and high boiling point
- d. low melting point and low boiling point
Insight: Ionic compounds typically exhibit high melting and boiling points due to the strong electrostatic forces between ions.
-
Valency is based on the number of...
- a. electrons
- b. nuclei
- c. neutrons
- d. protons
Insight: Valency is determined by the number of electrons in the outer shell that can be gained, lost, or shared to attain stability.
-
What is a negative ion?
- a. an atom that has gained a proton
- b. an atom that has gained an electron
- c. an atom that has lost a proton
- d. an atom that has lost an electron
Insight: Negative ions, or anions, form when an atom gains one or more electrons.
True or False
-
An atom with one or more additional electrons is called a cation.
- False - A cation is an atom that loses electrons and gains a positive charge.
-
When two or more elements bond they form a compound.
- True - A compound results from the chemical bonding of two or more different elements.
-
An ionic bond is formed when atoms share their electrons.
- False - Ionic bonds form from the transfer of electrons, not sharing; covalent bonds arise from sharing electrons.
-
Elements which lose or gain electrons easily tend to form covalent bonds.
- False - Elements that easily gain or lose electrons tend to form ionic bonds.
-
The chemical name of water is hydrogen oxide.
- True - Water, H₂O, can be referred to chemically as hydrogen oxide.
Table Analysis
Valency Table
| Periodic Table Group | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
Insight: Valency indicates an atom's ability to bond, often mirroring its group number for Groups 1-4, and diminishing as you move towards Group 8, where noble gases reside with a valency of 0.
Questions
-
Explain the relationship between valency and the number of electrons in an atom.
- Valency is the measure of an atom’s capacity to bond with other atoms. This is directly related to the number of electrons in its outermost shell.
-
Explain why noble gases have a valency of 0.
- Noble gases possess a complete valence shell, making them chemically stable and non-reactive under ordinary conditions, hence a valency of 0.
-
What is the formula for:
- Barium chloride?: BaCl₂
- Potassium oxide?: K₂O
- Aluminium oxide?: Al₂O₃
These exercises test understanding of fundamental chemistry concepts like atomic structure, bonding, and chemical nomenclature.
Extended readings: